Snow joke – January’s Ice structure, Ice Ih
What does it look like?
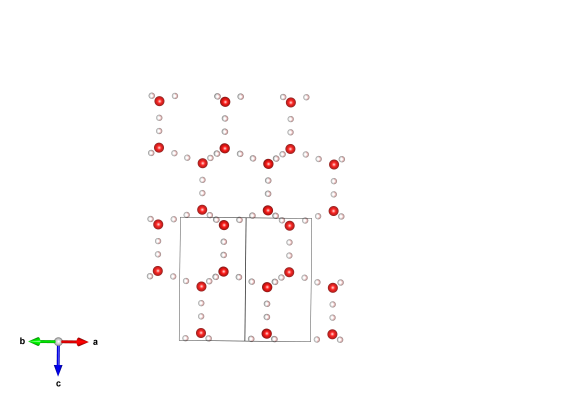
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
You see this every time you open your freezer, and often when you step outside on a winter’s day. This is the atomic arrangement of solid water that we are all most familiar with, known as hexagonal ice, or Ice Ih. It is the 6-fold symmetry in this arrangement of the water molecules that means that they can build into beautiful snowflakes.
There are a number of things that are remarkable in this structure, one is that the water molecules are never still – they are constantly flipping, with their hydrogens jumping from one position to another. This flipping, or disorder, is thought to be responsible for some of the remarkable properties that water and ice have, like the fact that ice floats on water. This means that to represent the structure we have to say that each of the hydrogen atoms are only occupied 50% of the time. You can find lots more about the Ice Ih structure here.
The name of this structure, Ice Ih, is a clue that water-ice can form lots of different structures; in fact there are currently 21 distinct solid forms of water. We’ll be picking one a month to write about, so stay tuned to find out more ice structures.
Where did the structure come from?
Seeing where hydrogen atoms are is really tricky with the conventional X-rays that most researchers use to investigate crystal structure. This is because the interaction with X-rays is dependent on the number of electrons an element has, and hydrogen only has one electron! So to discover this crystal structure Peterson and Levy used neutron diffraction, as neutrons don't interact with the electrons but the nucleus of an element itself, and in fact can interact with hydrogen quite well. This structure is #1008748 in the Open Crystallographic Database.






