Silicon – an element that is everywhere!
What does it look like?
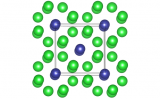
Silicon crystallises in a tetragonal, diamond structure, with space group Fd-3m.Image generated using Crystal Explorer 3.1, using data from the ICSD. Structure ID 60385.
What is it?
Silicon, element 14 of the Periodic Table, was first characterised in its pure form in 1823 by Swedish chemist Baron Jons Jakob Berzelius, although its existence was first hypothesised by Antoine Lavoisier as early as 1787. Its name stems from the Latin Silex, meaning hard stone, relating to its strong but rather brittle nature. It is the 8th most common element in the universe (by weight) and 2nd most common in the Earth's crust, with over 90% of the lithosphere composed of silicate minerals.
At room temperature, silicon can exist in two allotropes: amorphous and crystalline.
Silicon single crystals are grown using the Czochralski process and have a greyish, metallic appearance, whereas amorphous silicon is a brown powder. When doped with elements such as phosphorus, boron or arsenic, crystalline silicon forms the basis for many of today's solid state electronics, such as microchips, transistors, solar cells, and rectifiers. Other uses for silicon include fine chemical manufacture, aluminium casting and in steel refining, where it can improve ferritic phase hardening, resulting in harder and stiffer steels, and larger grain sizes leading to greater magnetic permeability. In biology, silicon is also essential for the synthesis of elastin and collagen, vital structural proteins used in the human body. By analogy with carbon, silicon is thought to have similar enough properties that would make silicon-based life possible and the study of hypothetical silicon-based lifeforms forms the basis of Silicon biochemistry.
Silicon can also form many other useful compounds: silica (SiO2) and silicates (SiO44-) are commonly found in ceramics, cement, glass and bricks. Silicon carbide (SiC) is used as an abrasive due to its extreme hardness and silicene, a 2D allotrope of silicon, is similar in structure to graphene. Silicon can also be polymerised with oxygen to form silicones and used in adhesives, sealants, medical implants and explosives.
Where did the structure come from?
The above structure was taken from data in the Inorganic Crystal Structure Database (ICSD). Structure ID 60385.