A ring of progress – Benzene
What does it look like?
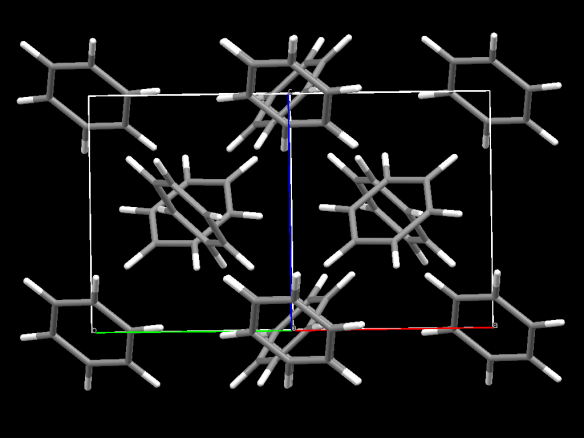
Image generated by the Mercury crystal structure visualisation software http://www.ccdc.cam.ac.uk/Solutions/CSDSystem/Pages/Mercury.aspx
What is it?
Benzene is the simplest aromatic hydrocarbon and also quite the crystallographic puzzle for many years. Though it had been known for a long time that benzene was made up of a ring of carbon atoms, in the early years of crystallography one question that was sought by a number of researchers was to determine the shape of the ring of benzene. There was a lot of debate, for instance W. L. Bragg (one of the founders of the field of crystallography) thought the molecule itself was bent.
The work of Cox in 1932 showed that the molecule within solid benzene was not bent and was in fact a flat ring. However, it was Kathleen Lonsdale who had paved the way for the understanding of the shape of the benzene molecule, with her structure of hexamethylbenzene in 1929. Together this work paved the way for the wide field of molecular crystallography that has many impacts on our way of life.
Where did the structure come from?
Cox first published the structure of benzene in the Proceedings of the Royal Society A in 1932, but refined his structure with a number of other investigations at different temperatures.