Do try this in your own home! Copper sulfate pentahydrate
What is it?

Image of a copper sulfate pentahydrate crystal, by Stephanb from http://en.wikipedia.org/wiki/ File:Copper_sulfate.jpg
Who amongst us has not, as a kid, grown single crystals of copper sulfate from a Home Science Kit, or even by pilfering the ingredients from your parents' garden shed? After a few trials you learn the knack of controlling the concentration and the location (i.e. temperature) of the solution of blue powder in water that will produce a large multifaceted single crystal with few fellow travellers. The surface of a dry crystal may begin to decompose within a few days, but you know you can always regenerate the lustre with further immersion in the solution.
Where did the structure come from?
We do not know if any of Walter Friedrich's, Paul Knipping's, or Max von Laue's parents showed them how to produce single crystals of copper sulfate this way, but we are very pleased that the young scientists had at least one good crystal for the historic recording of the first diffraction by X-rays in 1912 [1].
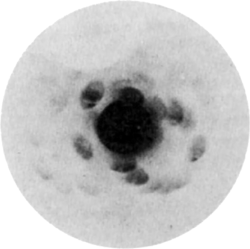
The first diffraction pattern, from copper sulfate pentahydrate, taken by Laue's group.
The first X-ray Laue pattern had just four obvious Laue spots. Improvement to the optics increased this to 20 or so within a short time, but still hardly enough unique observations to yield a definitive crystal structure. Fast forward some 20 years and monochromatic X-ray techniques had improved to the point where such 'complicated' structures could be solved, often by recourse to elegant chemical and physical arguments that nowadays are usually neglected in favour of brute-force numerical computation. Charles Beevers and Henry Lipson's description of the solution of the crystal structure of CuSO4.5H2O is a joy to read [2].
That was not the full story though. Copper sulfate hydrate, or to use its mineral name, chalcanthrite, crystallises as a pentahydrate, and the hydrogen atoms of the five water molecules were not visible in the X-ray studies of the 1930s. Another three decades were to pass before Bacon and Curry (we kid you not, that was their names!) located the hydrogen atoms by neutron diffraction [3].
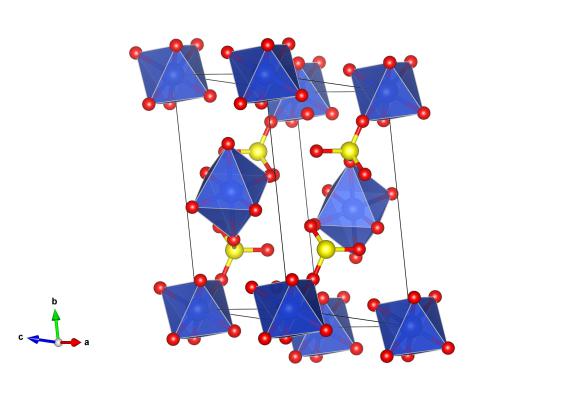
The crystal structure of copper sulfate pentahydrate, without the hydrogen positions as determined by Beevers and Lipson, Crystallography Open Database #1010527. The blue atoms are copper, the red oxygen and the yellow are sulfur. Image generated by the VESTA (Visualisation for Electronic and STructural Analysis) software http://jp-minerals.org/vesta/en/
You might think that interest in copper sulfate should have waned by now, but no! Just last year Martin Mourigal and coworkers explored the low-dimensional magnetism in a visually elegant experiment that is the envy of many physicists [4].
[1] Interferenzerscheinungen bei Röntgenstrahlen, W. Friedrich, P. Knipping, & M. Laue, Ann. Physik, 346 (1913) 971.
[2] The crystal structure of copper sulphate pentahydrate, CuSO4.5H2O, C.A. Beevers & H. Lipson, Proc. R. Soc. London Ser. A, 146 (1934) 570.
[3] The water molecules in CuSO4.5H2O, G.E. Bacon & N.A. Curry, Proc. R. Soc. London Ser. A, 266 (1962) 95.
[4] Fractional spinon excitations in the quantum Heisenberg antiferromagnetic chain, M. Mourigal, M. Enderle, A. Klöpperpieper, J.-S. Caux, A. Stunault & H. M. Rønnow, Nat Phys. 9 (2013) 435.