Biological structures
Posted on 01/04/2014

Julia Archbold
Cold as Ice… with 'Maxi' sacrifice
What is it?
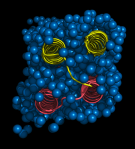
This is the structure of the antifreeze protein (AFP) Maxi. Maxi is found in winter flounders, fish that live in the cold North Atlantic ocean and the protein stops these fish from freezing. AFPs bind to ice crystals, and stop them from growing further. Usually, AFPs have residues on their outer surface that are responsible for interacting with ice crystals. However, Maxi is the first AFP that has its ice binding residues on the INSIDE of the protein.
What does it look like?
Unlike most globular folded proteins, that fold up tightly and repel waters from the centre of the protein, the Maxi protein is full of a layer of water molecules (http://www.rcsb.org/pdb/explore/explore.do?structureId=4KE2). Maxi has two rod shaped coils (yellow and pink) that pack loosely, loose enough to allow a single layer of water molecules to fill the spaces (blue spheres). The waters act as a sort of glue, holding the four helices of the Maxi together. These water molecules also 'leak' out of the ends of the gap between the helices, helping the protein to attach to a forming ice crystal and then stopping its growth.
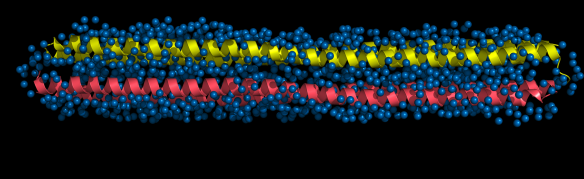
Image of the hyperactive type I antifreeze protein, Maxi (pdb code 4EK2) generated by Pymol (http://www.pymol.org/)
Where did the structure come from?
 The structure was published in Science in February 2014 by Peter Davies and colleagues (http://www.sciencemag.org/content/343/6172/795) (DOI: 10.1126/science.1247407).
The structure was published in Science in February 2014 by Peter Davies and colleagues (http://www.sciencemag.org/content/343/6172/795) (DOI: 10.1126/science.1247407).
Related articles
|
GOLD! The crystal structure of success |
A tree bark suspension and a Nobel Prize later … We wonder less about the structural basis of the action of aspirin |
Roses are red, violets are blue. Oxytocin in my brain makes me only want to be with you… |