Historic structures
Posted on 01/01/2014

Helen Maynard-Casely
The structure that started it all – Rock salt
What does it look like?
What is it?
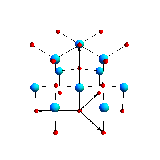
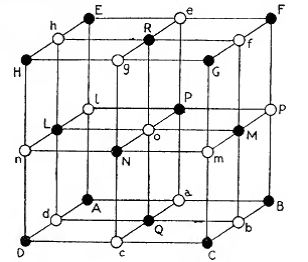
One of the crystal structures that started everything and you'll always have it in your kitchen. The structure of sodium chloride rock salt (or NaCl), was one of a handful of structures that W. L. Bragg presented to the Cambridge Philosophical Society on the 11th November 1912. This was the first time scientists had used diffraction (the patterns of scatter from crystalline solids discovered by Max von Laue). In the image above (taken from the book W. L. Bragg wrote with his father W. H. Bragg, X-rays and Crystal Structure) the black circles represent sodium atoms and the while chloride atoms. It is a very simple structure, in a cubic unit cell where each of the repeating lengths are 5.6 Å, and can be described with just two atoms.
Where did the structure come from?
We took this image of the structure of rock salt straight from W. L. and W. H. Bragg’s book, which is available at the open library here.
Related articles
|
Classical crystal structures – Wurtzite |
Seasons greetings. The crystal structure of Cocoa Butter |
Shrinking in the heat – lanthanoid hexacyanidocobaltates, a.k.a. LnCo(CN)6 |