Rigidity in carbon and hydrogen – Adamantane
What does it look like?
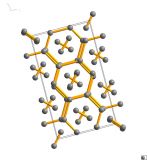
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
Adamantane is made of just two atoms, carbon (black) and hydrogen (white). Each adamantane molecule is constructed from four cyclohexane rings.
What is it?
Adamantane is a member of the cycloalkane family of hydrocarbons. Unlike many members of the cycloalkanes its molecular structure is very stable but also very rigid.
This structural rigidity is a result of the carbon-carbon bonds which adopt the same arrangement as those found in diamond. This resemblance is the root of the name, which is derived from Greek adamantinos meaning 'of diamond'.
The molecular structure of adamantane was first suggested by H. Decker in 1924; much to his surprise it had not yet been synthesised. (H 1924) The synthesis was attempted by several notable figures in organic chemistry and it was V. Prelog in 1941, building on the pioneering work of H. Meerwein, who finally succeeded.
Adamantane is one of the archetypal diamondoids; molecules that resemble diamond but typically of a fixed size or with other atoms substituting carbon. These structures are attractive possible building blocks for the construction of nanotechnology components; although due to their small size they remain relatively underused by jewellers.
Where did it come from?
The structure of adamantane was first determined by C. E. Nordman and D. J. Schmitkons in 1965, the data used in this article comes from work by J. P. Amoureux and M. Foulon. (P. and M. 1987) (E and J 1965)
Bibliography
E, Nordman C, and Schmitkons D J. Acta Crystallogr. 18 (1965): 764.
H, Decker. "Versammlung deutscher Naturforscher und Ärzte. Innsbruck, 21–27 September 1924." Angew. Chem. 37, no. 41 (1924): 795.
P., Amoureux J., and Foulon M. Acta Crystallogr. 43 (1987): 470.