How to Grow Crystals
The idea is to grow a single crystal, not a bunch of crystals. You will first need to grow a small perfect crystal, your seed crystal, around which you will later grow a large crystal. It is therefore essential to avoid excessive rapid growth, which encourages the formation of multiple crystals instead of a single crystal.
What You Need...
- Substance to be crystallized
- Distilled or demineralized water
- A small wood rod, popsicle or sate stick
- A shallow dish (e.g. Petri dish)
- Thermometer
- Balance
- Plastic or glass container
- Heating plate
- Beaker of 2 to 4 litres volume
- Fishing line (1 to 2 kg strength)
- Superglue
- Styrofoam box or picnic cooler
- A magnifying glass
Important Things to Know in Advance!
- How much substance you have to work with, which you can determine by weighing it on a balance.
- The solubility of the substance in water at room temperature, which you can obtain from a chemistry reference book.
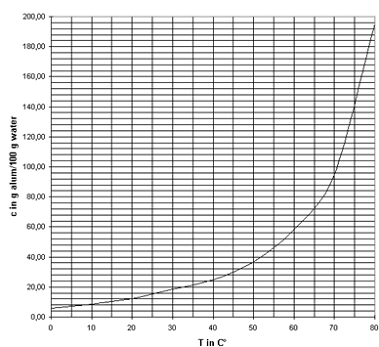
- It would also be useful to know the solubility of the substance at elevated temperatures, which is information that may also be available in a reference book such as Handbook of Chemistry and Physics. Figure 1 shows the solubility of alum as function of the temperature.
Of course, you need to make a careful assessment of any risk or safety precautions associated with the material you plan to use. For example,
- check the safety data sheet supplied with any chemicals;
- ensure that children are aware of the dangers of hot plates, or of mounting materials such as superglue;
- use safety glassware and other appropriate laboratory equipment;
- provide lab coats, gloves and safety goggles as appropriate.
The protocol!
First Stage: Grow a Seed Crystal
- Warm about 50 mL of water in a glass container.
- Dissolve a quantity of the substance to produce a saturated solution at the elevated temperature.
- Pour the warm solution into a shallow dish.
- Allow the solution to cool to room temperature.
- After a day or so, small crystals should begin to form as in Figure 2.
- Remove some of the crystals.
- With a magnifying glass select a beautiful and transparent small crystal. This will be your seed crystal. Weigh the crystal.
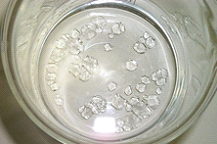
Figure 2. Seed crystals of alum. (Credit: picture by Luc Van Meervelt)
Second Stage: Grow a Large, Single Crystal
- Glue the seed crystal at the end of a piece of fishing line by using superglue (be careful not to glue your fingers together!).
- Check with the magnifier that the seed crystal is well-fixed to the line.
To grow your large, single crystal, you will need a supersaturated solution.
The amounts of substance and water to be used will depend upon the solubility at room and elevated temperatures. You may have to determine the proper proportions by trial and error (just like the first scientists did).
- Place about double the amount of substance that would normally dissolve in a certain volume of water at room temperature into that volume of water. (e.g. If 30 g of X dissolves in 100 mL of water at room temperature, place 60 g of X in 100 mL of water.) Adjust the proportions depending upon how much material you have. Use clean glassware.
- Stir the mixture until it appears that no more will go into solution.
- Continue stirring the mixture while gently warming the solution.
- Once all of the substance has gone into solution, remove the container from the heat.
- Allow the solution to cool to room temperature.
- You now have a supersaturated solution. Allow to cool to room temperature.
- Carefully suspend your seed crystal from the stick into the cold supersaturated solution in the middle of the container with supersaturated solution (Figure 3).
- Cover the container in which the crystal is growing with plastic wrap, aluminum foil or a piece of cardboard in order to keep out dust, and reduce temperature fluctuations.

Figure 3. Seed crystal of alum suspended in saturated solution. (Credit: picture by Luc Van Meervelt)

Figure 4. Styrofoam or isomo box.
- Observe the crystal growth. Depending upon the substance, the degree of supersaturation and the temperature, this may take several days before the growth slows down and stops.
- Resupersaturate the solution. This may need to be done on a daily basis, especially when the crystal gets larger. But first, remove the crystal.
- Each time the solution is saturated, it is a good idea to ‘clean’ the monocrystal surface, by
-
- making sure the crystal is dry;
- not touching the crystal with your fingers (hold only by the suspending line if possible);
- removing any ‘bumps’ on the surface due to extra growth;
- removing any small crystals from the line.
- Resuspend the crystal back into the newly supersaturated solution.
- Repeat the previous steps as needed.
Some FAQ's...
Why does the crystal stop growing?
A crystal will only grow when the surrounding solution is supersaturated with solute. When the solution is exactly saturated, no more material will be deposited on the crystal. (This may not be entirely true. Some may be deposited, however an equal amount will leave the crystal surface to go back into solution. We call this an equilibrium condition.)
Why did my crystal shrink/disappear?
If your crystal shrank or disappeared, it was because the surrounding solution became undersaturated and the crystal material went back into solution. Undersaturation may occur when the temperature of a saturated solution increases, even by only a few degrees, depending upon the solute. (This is why temperature control is so important.)
How do I get crystal growth restarted?
Make the solution again supersaturated!
Help, my crystal loses its transparency!
When removing the crystal from the solution, clean it very quickly in water to rinse the thin layer of solution on the crystal surface away. Otherwise this thin layer would leave an amorphous precipitate on the surface after evaporation. This will decrease the transparency of the crystal, and you will not be able to harvest a perfect transparent crystal as in Figure 5.
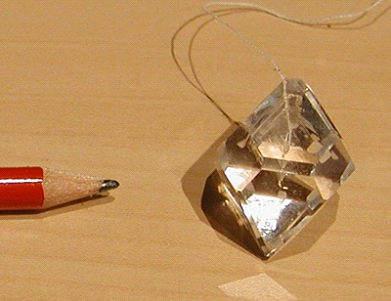
Figure 5. Transparent alum crystal. (Credit: picture by Luc Van Meervelt)
What is the difference between an undersaturared, saturated and supersaturated solution?
In recrystallization, one tries to prepare a solution that is supersaturated with respect to the solute (the material you want to crystallize). There are several ways to do this.
One is to heat the solvent, dissolve as much solute as you can (said to be a "saturated" solution at that temperature), and then let it cool. At this point, all the solute remains in solution, which now contains more solute at that temperature than it normally would (and is said to be "supersaturated").
This situation is somewhat unstable. If you now suspend a solid material in the solution, the "extra" solute will tend to come out of solution and grow around the solid. Particles of dust can cause this to occur. However this growth will be uncontrolled and should be avoided (thus the recrystallization beaker should be covered). To get controlled growth, a "seed crystal", prepared from the solute should be suspended into the solution (Figure 6).
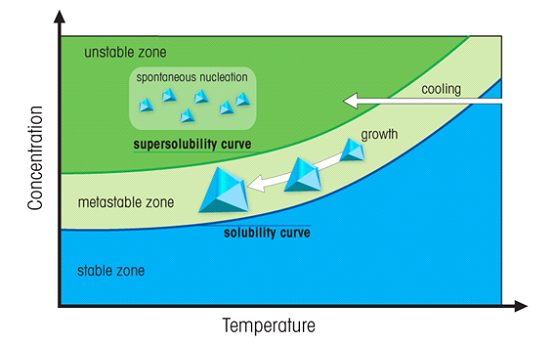
Figure 6. The region above the solubility curve is the called "supersaturated". In the unstable zone (green) spontaneous nucleation occurs. A crystal suspended in the metastable zone will grow further.
The supersaturation method works when the solute is more soluble in hot solvent than cold. This is usually the case, but there are exceptions. For example, the solubility of table salt (sodium chloride) is about the same whether the water is hot or cold.
Can I prepare a supersaturated solution in a different way?
A second way to get supersaturation is to start with a saturated solution and let the solvent evaporate. This will be a slower process. A third method is given below:
- Select an appropriate volume of water.
- Warm this water to about 15–20 deg above room temperature.
- Add some of your substance to the warm water and stir the mixture to dissolve completely.
- Continue adding substance and stirring until there is a little material that won’t dissolve.
- Warm the mixture a bit more until the remaining material goes into solution.
- Once all of the substance has gone into solution, remove the container from the heat.
- Allow the solution to cool to room temperature.
- You now have a supersaturated solution.
I am a perfectionist, what can I do additionally?
To get improved symmetry and size, slowly rotate the growing monocrystal (1 to 4 rotations per day). An electric motor with 1 to 4 daily rotations might be difficult to find (consider one from an old humidity drum-register or other apparatus). This option becomes useful only when a monocrystal gets rather big. You can also place the beaker into a thermostated bath set to a few degrees above room temperature.
Slow or fast growing, what is the best?
The rate at which crystallization occurs will affect crystal quality. The more supersaturated a solution is, the faster growth may be. Usually, the best crystals are the ones that grow slowly.
What is the effect of impurities?
Once you have mastered the crystal growth, you may be interested in trying to grow single crystals in the presence of introduced ‘impurities". These impurities may give different crystal colours or shapes.
Does this method also work for proteins?
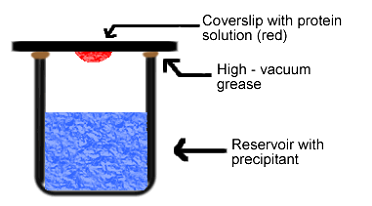
No, it is not possible to make a supersaturated protein solution by dissolving protein into a hot solvent. The protein will denaturate and loose its regular folded structure. A special set-up is needed here. In the hanging drop method (Figure 7) for example, a droplet containing protein, buffer and precipitant is hanging above a larger reservoir containing buffer and precipitant in a higher concentration. As water evaporates from the droplet it will transfer to the reservoir where it is bound to the precipitant. During this process the protein is concentrated. Once supersaturation is reached, nucleation and crystal growth is starting.