Crystals and X-rays: made for each other!
the ideal tool for studying crystalline materials

Wilhelm Conrad Röntgen, Nobel Prize 1901; and his laboratory
© Deutsches Röntgen Museum
X-rays: In 1895, Röntgen discovered a new type of radiation but was unable to determine its precise nature. In the end, he gave up and called them X-rays. Invisible and able to pass through solid matter, these rays were studied by scientists from Australia, Britain and Germany who used crystals to understand their properties. In 1912, von Laue, Friedrich & Knipping exposed a crystal to X-rays. The experiment, now called diffraction, was initially carried out in order to understand the nature of the radiation; instead, its real importance was to reveal the regular order and symmetry of the crystals themselves. This led to the extraordinary possibility of determining the internal atomic arrangement of all crystals.

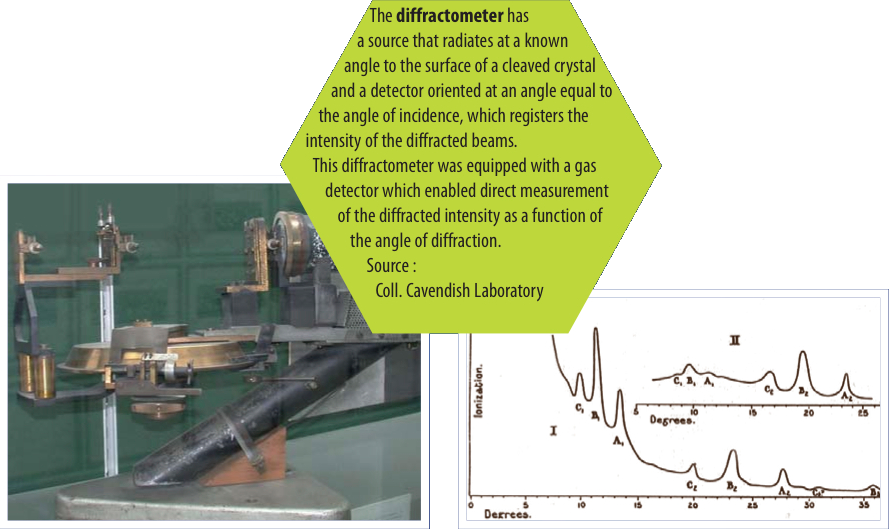
William Lawrence Bragg and his father, William Henry Bragg, realized that X-rays could be used to understand crystals, to "see" their inner structure and thus developed the new science of X-ray crystallography. W. L. Bragg is most famous for his law on the diffraction of X-rays by crystals, made during his first year as a research student in 1912. Bragg’s law made it possible to calculate the positions of the atoms within a crystal. The "diffraction" of X-rays thus changed from the status of being a physical phenomenon to that of a tool for exploring the arrangement of atoms within crystals. This discovery led to an intense period of research. Most of these pioneering scientists received Nobel prizes, including the first Australian, and the youngest ever, Nobel Prize winner, W. L. Bragg.
In the 19th century, German and French researchers introduced the concept of symmetry to classify crystals. They used mathematics to formalize their classification theory. Thus, by the beginning of the 20th century, even without being able to "see into" a crystal, crystallographers had developed the notion of atomic order and periodic repetition to understand both the external form of crystals as well as their symmetry.

William Lawrence Bragg (left); William Henry Bragg (right):
joint Nobel Prize 1915



