The structures behind birthstones – January Garnets
What does it look like?
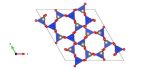
This picture was drawn using Diamond structure visualisation software. Si is white, O is grey and Fe and Al are green.
What is it?
 Garnet is the first of our Birthstones. It has been known since at least Roman times with the name garnet coming from the Latin "Granatus" meaning "Grain-like". Most people know it as a red stone which can either be worn as a rough stone or a cut gem. In fact, garnet displays the widest variety of colour variations of any mineral. This is due to the large number of different elements which can substitute into the garnet structure.
Garnet is the first of our Birthstones. It has been known since at least Roman times with the name garnet coming from the Latin "Granatus" meaning "Grain-like". Most people know it as a red stone which can either be worn as a rough stone or a cut gem. In fact, garnet displays the widest variety of colour variations of any mineral. This is due to the large number of different elements which can substitute into the garnet structure.
Garnet is a silicate mineral. This means that it contains silica tetrahedra within the structure. These are the building blocks of many common rock forming minerals. They are formed from Silicon and Oxygen (see image) and can be either isolated tetrahedra or more complex arrangements of chains or nets of tetrahedra. In both cases, Other elements (cations) are arranged in the gaps between the tetrahedral. For garnet the most common garnet is one which contains iron and aluminium in addition to the silicon and oxygen. It is the iron and aluminium which substitute to form the coloured varieties of garnet.
One of the rarest garnets is the green/blue garnet Knorringite. The colour is a result of Magnesium and Chromium within the structure in place of the more common Iron and aluminium (respectively). The presence of this variety of garnet is an indicator for the presence of diamonds in the rock and so a find of such garnets is very sought-after!
Where did the structure come from?
The structure was first determined in 1924 by Menzer using X-ray diffraction. This was the first application of X-ray crystallography to mineralogy. It was part of a coordinated project in the 1920s to investigate the structures of a range of silicate minerals which also included Olivine and Pyroxene. Since then it has been redetermined by many people using many different techniques. As it is a relatively simple and well known structure, it makes a good sample to try things out with.