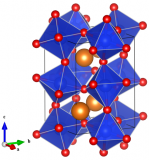
An Earth and Mars mineral – Meridianiite MgSO4.11H2O
What does it look like?
The structure of Meridianiite at 250 K. This image was created using the Diamond visualisation software. Mg is pinky-red, S is yellow, O is white and hydrogen (technically deuterium), is grey.
What is it?
MS11, Meridianiite, is another lovely (in the opinion of the author), salt hydrate, just like Mirabilite and Epsomite. Like those salts, it is also thought to be a key rock-forming mineral on the icy satellites of Jupiter. But hang on, how come it is named after a place on Mars? Well, it is there too … probably.
MS11 is only stable below 2°C. This means that on Earth we mainly find it at the cool extremes of our planet; tundra and cold deserts are MS11's favourite places to be. On Mars the temperature variations on the planet mean that the conditions that MS11 forms in and is stable at are much more prevalent and the "Mg-sulphates" which have been identified by various instruments on Mars may well be Meridianiite.
As we heard with Bridgmanite, a mineral can only be named once it has been found in nature. This mineral was first synthesised by Fritzsche in 1837. Just like Glauber and Mirabilite, this salt was called Fritzsche's salt to begin with. Fritzsche thought that MS11 was actually MS12 – Magnesium sulphate with 12 water molecules attached rather than 11. He worked out the composition by measuring the mass lost as the compound dehydrated and must have counted 12 waters instead of 11. This was put right in 2006 by Petersen and Wang who solved the structure and realised there were only 11 waters rather than 12. Shortly after this, a natural sample of Meridianiite was found in Canada and so it was officially named and became a fully paid up mineral.
Where did the structure come from?
This structure is actually for deuterated Meridianiite, (the hydrogen atoms are replaced by deuterium) and comes from a study using time-of-flight neutron diffraction to investigate how the structure of MS11 changes as temperature varies from 10 – 300 K.
Fortes A. D., Wood I. G. and Knight K. S. (2008) The crystal structure and thermal expansion tensor of MgSO4-11D2O (meridianiite) determined by neutron powder diffraction. Physics and Chemistry of Minerals 35 207-221 and is available in the American Mineralogist Crystal Structure Database.