A bit of a mouthful – hexamethylenetetramine
What does it look like?
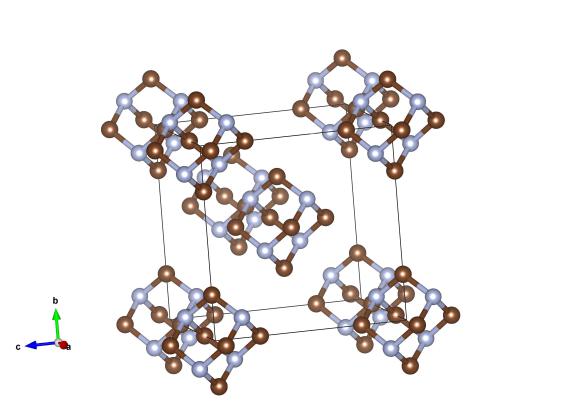
Here the brown atoms are carbon, and the light blue atoms are nitrogen atoms. Image generated by the VESTA (Visualisation for Electronic and STructural Analysis) software http://jp-minerals.org/vesta/en/
What it is?
Though its name is a bit of a mouthful, this is a relatively simple molecular compound. It is made up of six carbon atoms and four nitrogen atoms which bond together to form a little cluster.
The structure of hexamethylenetetramine is significant to crystallography. It was determined in 1923 and was arguably the first molecular structure to be found. It was chosen for the investigation beacuse it was known to form a cubic crystal structure, one of very few molecular materials to do so. Materials with cubic symmetry are more straightforward to solve, as there are fewer parameters to find. This work really proved that molecules would arrange into crystals, and paved the way for Kathleen Lonsdale's work on benzene ring. This, in turn, lead to the field of molecular crystallography which is still very active today.
Where did the structure come from?
This structure comes from the 1923 Journal of the American Chemical Society paper by Dickinson and Raymond. There's no crystallographic information file for this one in the Crystallography Open Database, so the coordinates were taken from the paper itself.