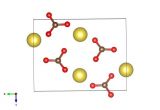
Absorbing and beautiful, zinc nitrate
What does it look like?
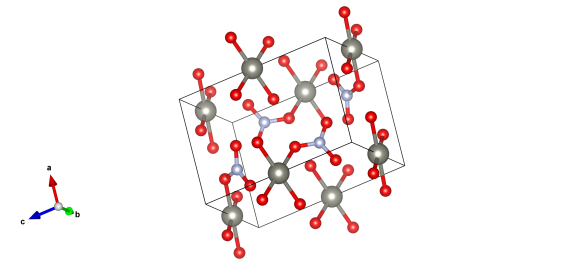
Zinc atoms are grey, nitrogen light blue and the red atoms represent oxygen (some of which will be the centre of water molecules.)
What is it?
Zinc nitrate is one of those materials that every chemist has in their cupboard. It's very deliquescent, meaning that it will absorb water from the atmosphere. The reason we chose to feature it today is that we stumbled upon this beautiful video, on the RI Channel.
Watch the full video with related content here: http://richannel.org/beautiful-chemical-reactions
This shows, among other things, beautiful zinc nitrate 'trees' growing – silver atoms are being substituted for zinc as the material is re-crystallising.
Where did the structure come from?
Given its affinity to water it's really very rare to crystallise pure zinc nitrate with no water molecules in the crystal structure. So we've featured zinc nitrate dihydrate (two waters for every zinc nitrate unit), a structure determined by D. Petrovic and B. Ribár in 1975.